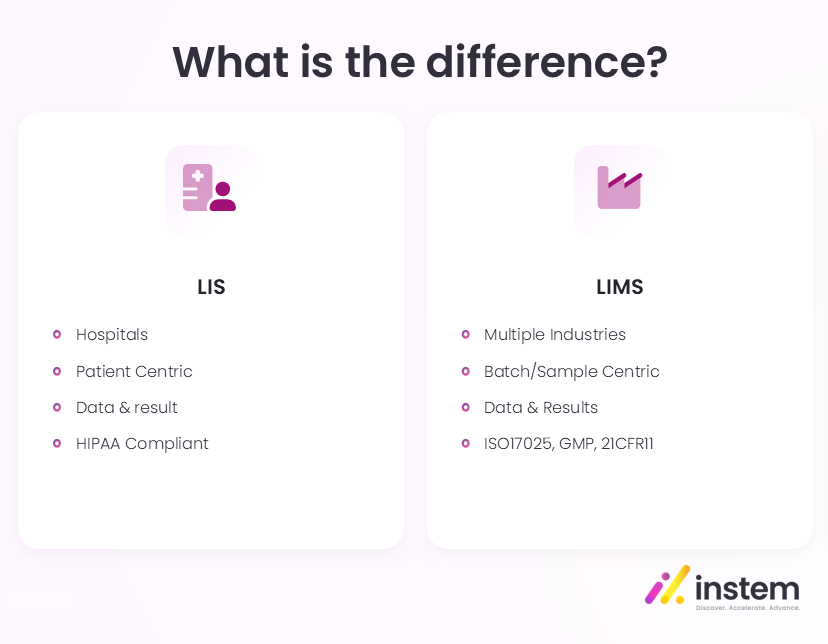
There is often some confusion in the use of the terms Laboratory Information System (LIS) and Laboratory Information Management System (LIMS), and a temptation to use the terms interchangeably. Generally, the term LIS refers to systems used to manage clinical diagnostic testing within a hospital or healthcare environment. LIMS, on the other hand, refers to systems used in other analytical testing environments, for example, those associated with pharmaceutical, food, or chemical manufacturing, environmental control, and commercial non-clinical testing organizations.
LIS
When comparing LIS vs LIMS, the traditional differences cited are that LIS is patient-centric with an emphasis on keeping personally identifiable information secure. This was formalized with the HIPAA requirements introduced in the USA in 1996. LIS stores and manages patients’ data and test results. LIS system software typically includes features such as:
Integration with numerous other health-based systems, potentially using protocols such as HL7
- Patient demographic information, including name, Date of Birth, ethnicity and blood type—key data that allows accurate interpretation of test results and diagnosis of conditions
- Tracking of patient samples and associated test results
- A historic record of clinical diagnosis, treatment and patient outcomes
- Integration of high-throughput clinical analyzers
- Integration with numerous other health-based systems, potentially using protocols such as HL7
LIMS
By contrast, LIMS are sample-centric. They have always been focused on efficiently managing a laboratory’s workflows, usually, but not always, in batch-oriented environments. LIMS supports organizations working to standards and regulations such as ISO/IEC 17025, setting out the competence requirements for testing and calibration; Good Manufacturing Practice (GMP), a code of practices controlling the manufacture of certain products like food and pharmaceuticals; and FDA 21 CFR Part 11, governing the use of electronic records and electronic signatures. A LIMS typically includes features such as:
Integration with other business systems such as Enterprise Resource Planning (ERP) Systems and Manufacturing Execution Systems (MES)
- Batch tracking and management, in addition to individual sample tracking
- Workflow management that ensures lab practices are adhered to
- Management of multiple specifications for product quality checking
- Laboratory management functionality, such as Instrument calibration and staff competency management
- Integration with analytical instruments and instrument systems from simple balances to complex data systems
LIS vs LIMS
A Laboratory Information Management System (LIMS) provides extensive benefits to regulated industries such as pharmaceuticals, food, and water testing. These benefits—secure data storage within a centralized repository, seamless integration with analytical instruments, and automated management reporting, including the generation of Certificates of Analysis—have driven widespread adoption of LIMS across a diverse range of industries. By enabling digital recordkeeping and automated workflows, LIMS solutions play a key role in supporting paperless operations and achieving the vision of a fully digital laboratory.
While there is considerable overlap between the functionality of a Laboratory Information System (LIS) and a LIMS, their focus and applications differ. Systems such as biobank management, clinical trial management, and veterinary diagnostics often appear similar to LIS solutions because they handle patient demographic data, clinical test results, and result interpretations. However, these systems are typically implemented using a LIMS platform.
This crossover highlights the inherent flexibility of modern LIMS platforms, which can be configured to support a wide variety of laboratory environments and data management requirements. Unlike LIS solutions, which are primarily designed for clinical and hospital-based workflows, LIMS can be adapted to research, quality control, manufacturing, and diagnostic settings, making it the preferred choice for organizations seeking a unified, configurable solution that can evolve with their operational needs.
Modern LIS vs LIMS Capabilities
Unfortunately, there is no universally accepted definition distinguishing the terms LIS and LIMS. Their interpretation often depends on an individual’s professional background and can vary across industries and even from country to country. The key distinction lies in their primary purpose: Laboratory Information Systems (LIS) are designed specifically for managing diagnostic testing, while Laboratory Information Management Systems (LIMS) are used for a broader range of testing and research applications.
Although LIS and LIMS share many similarities, they also differ in critical ways—making them far from interchangeable. These differences span workflow design, regulatory compliance requirements, supported industry standards, and overall cost. Understanding these distinctions is essential when selecting the most suitable solution for your laboratory.
Modern LIS and LIMS platforms have evolved to meet the diverse needs of both clinical and non-clinical laboratories. However, LIMS solutions demonstrate greater versatility. Because each industry operates under unique conditions, most LIMS providers develop specialized versions of their software to meet sector-specific requirements.
Instem’s LIMS solutions stand apart. All of our starter systems are built on the same robust software foundation, with no changes to the core code. Instead, workflows are customized using Matrix Gemini LIMS’ unique graphical configuration tools, allowing laboratories to adapt processes without programming. This approach simplifies maintenance and upgrades, streamlines support—even for customer-configured screens—and significantly reduces total lifetime ownership costs.
For further information or to request a demonstration, contact us today to discover how Instem’s LIMS solutions can streamline and future-proof your laboratory operations.


