Centrus®
Translating Data into Safer Clinical Outcomes
Centrus is a centralized platform for accelerating translational safety assessments. It combines computing modules with diverse clinical and non-clinical data sets to instill confidence in decision-making at all stages of the drug development process. Centrus is a unique solution for streamlining translational development, helping to ensure future success.
Start making smarter, safer decisions today!
What is Centrus?
Centrus is a cutting-edge platform that integrates advanced computational tools with extensive, high-quality data to accelerate and improve safety assessments in R&D. By leveraging preclinical data, Centrus helps researchers anticipate how potential therapies will perform in human trials, identifying safety risks early.
Toxicology testing is costly and time-consuming, and failures due to unforeseen toxicity during clinical trials can result in major delays and wasted resources. Centrus addresses this challenge by enabling early toxicology risk assessment, giving researchers a stronger foundation for success and reducing the chances of late-stage setbacks. With Centrus, predictions are based on the latest datasets, ensuring your conclusions align with the best available evidence.
Centrus also acts as a centralized repository for shared data, facilitating collaboration across teams and departments.

Key Benefits of Centrus
Saves Time
Centrus accelerates decision-making in R&D by enabling early detection of toxicology risks, reducing the need for time-consuming late-stage revisions.
Improves Safety
By predicting adverse effects before clinical trials begin, Centrus helps researchers make safer decisions. This reduces the risk of exposing patients to harmful compounds and strengthens confidence in therapeutic candidates.
Promotes Optimal Decision-making
Centrus provides access to a vast catalog of curated, high-integrity preclinical and clinical data, sourced from leading pharmaceutical organizations and public databases, ensuring high-quality decision-making.
Simplifies Collaboration
As a centralized platform, Centrus promotes seamless collaboration across departments and organizations, with shared data repositories and standardized formats.
Improves ROI
By identifying safety concerns earlier and reducing the likelihood of costly trial failures, Centrus helps maximize the value of your investment, supporting more efficient, successful development pipelines.
Core Features
Centrus is a powerful platform supporting R&D efforts by providing advanced tools for safety prediction and data integration.

Integrated Data Sources
Connects private, public, and shared databases in a secure, centralized location to provide a unified view of preclinical and clinical data. This speeds up decision-making and removes the need to query diverse sources or scattered data.

Predictive Toxicology Tools
Enables early identification of potential safety issues using advanced modeling and semantic linking.

SEND-Based Data Harmonization
Standardizes data to simplify interpretation, regulatory alignment, and cross-study comparisons. This promotes collaboration across teams and integration of datasets.

Interactive Data Visualization
Streamlined data visualization speeds up decision-making and helps researchers quickly spot trends and meaningful correlations.

Advanced Query and Filtering Apps
Tools like the Query Application and semantic service make it easy to translate, search, and filter findings across datasets.

Ethical Data Stewardship
Centrus facilitates safe, ethical data sharing by acting as a trusted data steward ensuring the use of the data is controlled by the platform.
Why Choose Centrus?
Centrus is a powerful platform designed for organizations of all sizes that want to harness diverse, high-value datasets to improve safety assessments. Unlike basic data storage or visualization tools, Centrus goes further by providing access to non-clinical toxicology data contributed by leading pharmaceutical companies, offering clients valuable insights drawn from some of the industry’s most trusted sources.
Centrus uses semantic modeling and advanced ontologies to intelligently link clinical and preclinical data. This enables researchers to predict potential adverse events and determine whether such events were previously indicated in preclinical studies, leading to more confident and data-driven decisions. By helping researchers identify safety concerns early, Centrus reduces costly late-stage setbacks, making it a smart, ROI-driven choice for teams focused on speed, accuracy, and success.
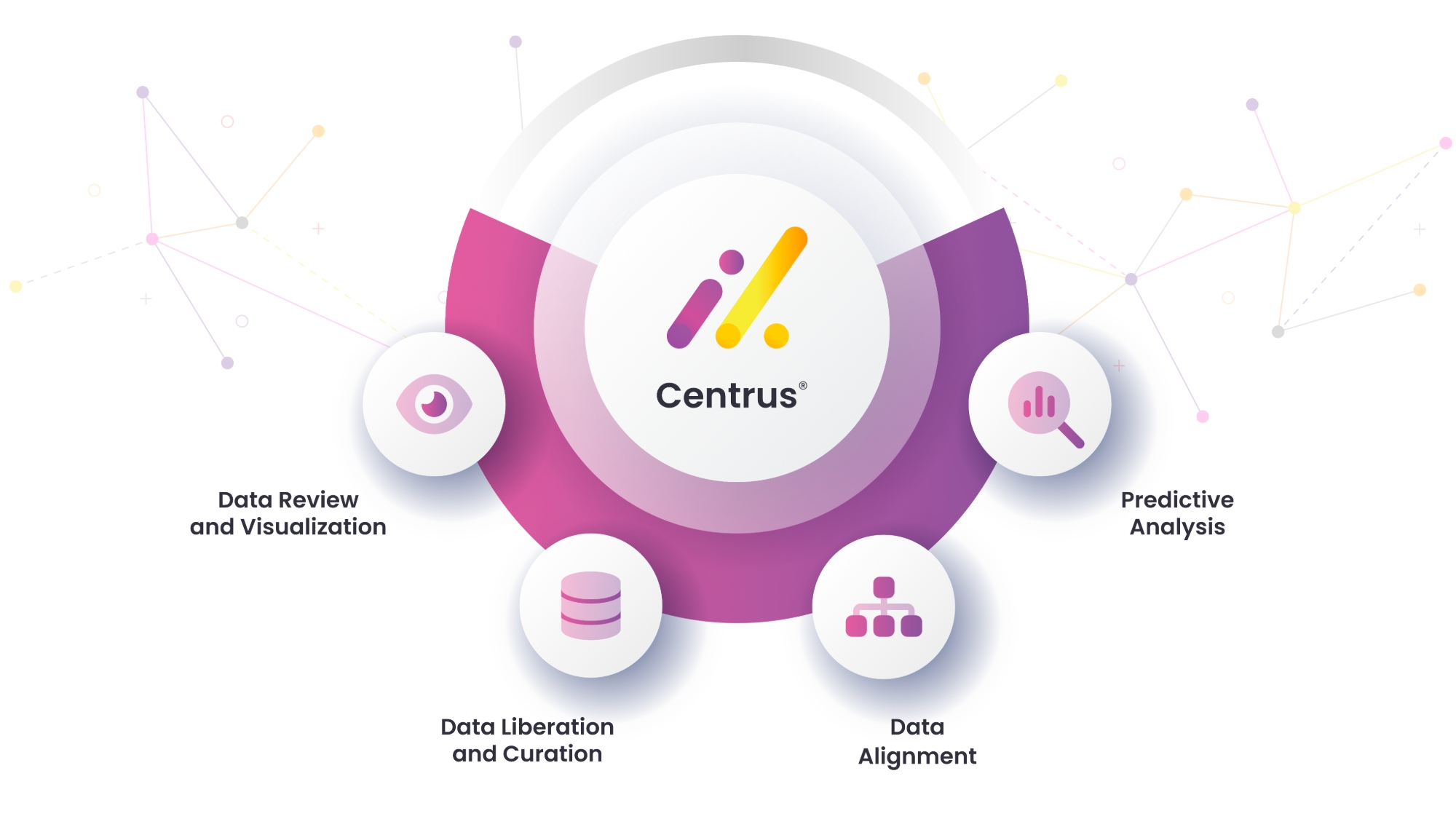
How It Works
Centrus simplifies and accelerates the drug development process by harmonizing diverse data sources for seamless integration and analysis. Through a powerful combination of data standardization, visualization, and predictive analysis, Centrus empowers teams to make informed decisions and drive safety outcomes earlier in the process.
Data Review and Visualization
By leveraging SEND standards and linked vocabularies, Centrus ensures data is harmonized, streamlining both integration and visualization processes.
Data Liberation
and Curation
Access diverse, high-quality data sources from pharmaceutical companies, as well as clinical and preclinical research data.
Data
Alignment
Centrus standardizes and integrates data from diverse sources into a centralized, shared database to ensure consistency, compatibility, and ease of analysis.
Predictive
Analysis
Leverage dedicated software to predict safety outcomes, identify risks earlier, and guide more effective decisions throughout the drug development process.
Frequently Asked Questions
Centrus unlocks advanced capabilities for toxicology scientists. Below are some of the most common questions we receive from scientists looking to upgrade their drug development processes.
What kind of support is available for Centrus?
Centrus is backed by Instem’s technology-enabled services and expert consulting, ensuring users receive guidance and support tailored to their research goals.
Can Centrus be used collaboratively across teams?
Yes. Centrus enables secure data sharing and centralized access. This allows cross-functional teams to collaborate efficiently while maintaining control over data privacy and compliance.
How is Centrus deployed?
Centrus is deployed using secure, scalable cloud technologies, allowing for seamless access, reliable performance, and easy integration.
What types of data can Centrus handle?
Centrus supports a wide range of data sources, including public, private, and shared datasets.
Get a Free Demo Today
Centrus empowers R&D teams to make faster, safer, and smarter decisions with high-quality data, predictive tools, and seamless collaboration. Don’t wait to modernize your drug development workflow.
