Leadscope Model Applier (LMA) is a computational toxicology tool designed to support regulatory-compliant risk assessment through predictive modeling. The latest release version 2025.0 introduces advanced features to enhance toxicity prediction, improve data integration, and support in silico safety assessments.
New Features and Enhancements
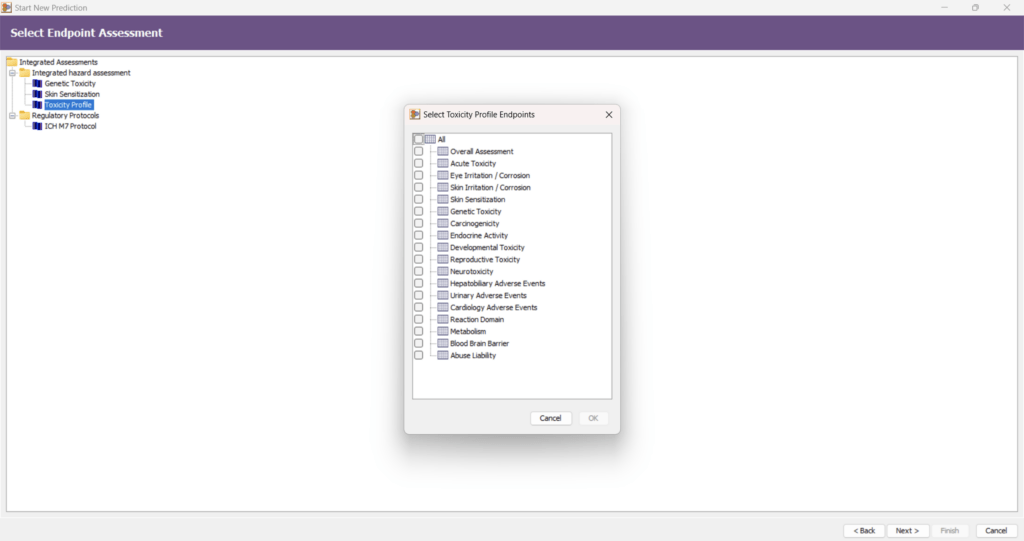
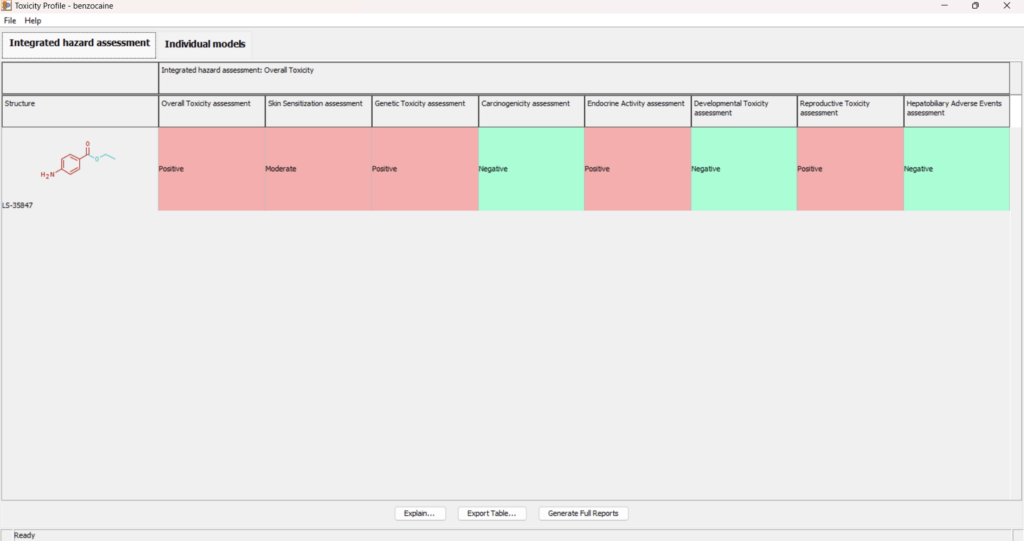
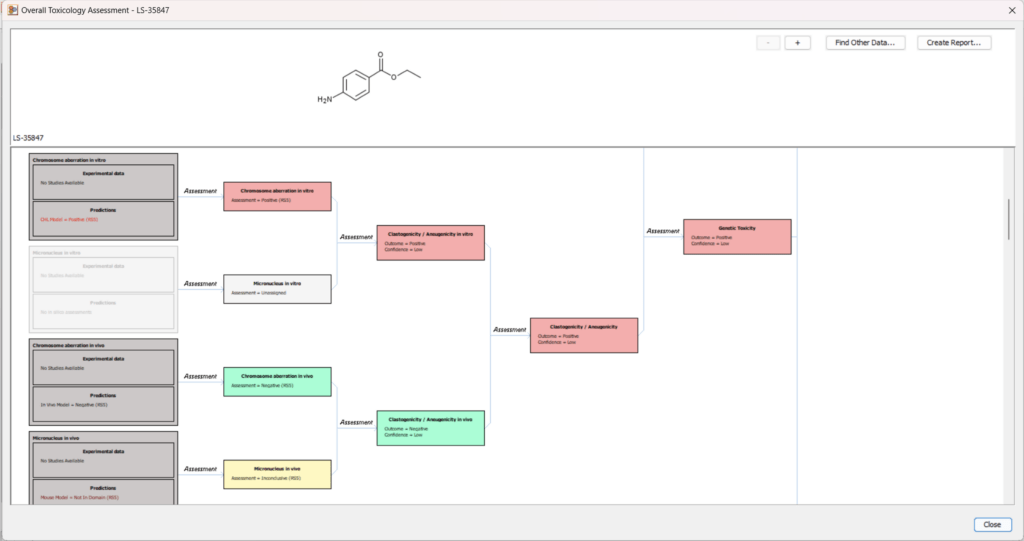
Toxicity Profiler
In silico profiling is a critical approach for understanding the toxicological characteristics of chemicals. The new Toxicity Profiler improves how data is summarized and organized, enabling a more structured interpretation of toxicological risk.
- Aggregates and integrates experimental data and computational predictions across different mechanisms and observed effects.
- Enhances early-stage screening in drug discovery by identifying potential toxicity concerns.
- Supports read-across assessments by identifying structural features associated with specific toxicity endpoints.
- Uses both statistical models and expert alerts to justify positive and negative predictions.
Database and Model Updates
Acute Toxicity Enhancements
To support regulatory toxicology and the 3Rs principles (replacement, reduction, refinement of animal testing), Leadscope has expanded its acute toxicity databases and models.
| Features | Description |
| Expanded dataset | Over two thousand new acute toxicity records from ECHA REACH dossiers. |
| New models | New CLP statistical models and alerts predicting rat acute oral toxicity. |
| Regulatory support | Experimental data and predictive models align with GHS and CLP classifications to support risk assessment. |
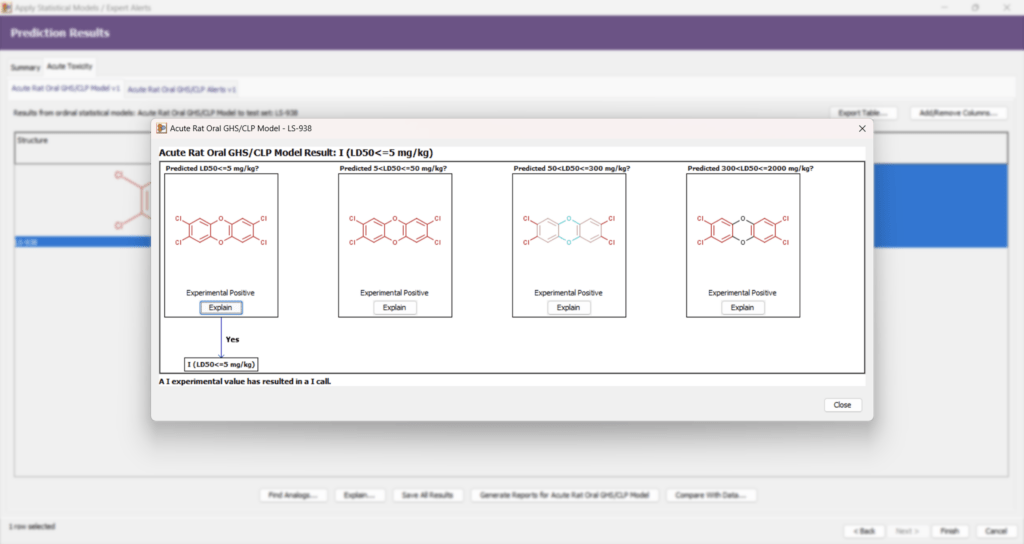
Skin Sensitization Model Enhancements
The skin sensitization model has been updated to improve the accuracy of predictions for regulatory applications, including extractables and leachables assessment, classification and labeling, and non-genotoxic impurity evaluations.
- Hundreds of new chemical structures, incorporated from ECHA REACH dossiers.
- Improved data transparency, including depicting inherited structure-activity relationship (SAR) calls.
- Updated models with refined mechanistic insights to enhance predictions.
Bacterial Mutation Alerts Update
- Improved mechanistic annotation for bacterial mutation predictions.
- Updated alert definitions to increase confidence in identifying mutagenic potential.
- Supports regulatory compliance with ICH M7 guidance.
N-Nitrosamine Read-Across Support
The Carcinogenic Potency Categorization Approach (CPCA) is a standard methodology used by health authorities to categorize n-nitrosamine impurities based on chemical structure and carcinogenic risk. Leadscope has now integrated CPCA within the Read-Across feature.
- Aligns with the CPCA calculator used by global regulatory agencies.
- Expands the ability to analyze n-nitrosamine compounds using read-across approaches.
- Includes local similarity analysis to improve analog identification and enhance confidence in acceptable intake calculations.
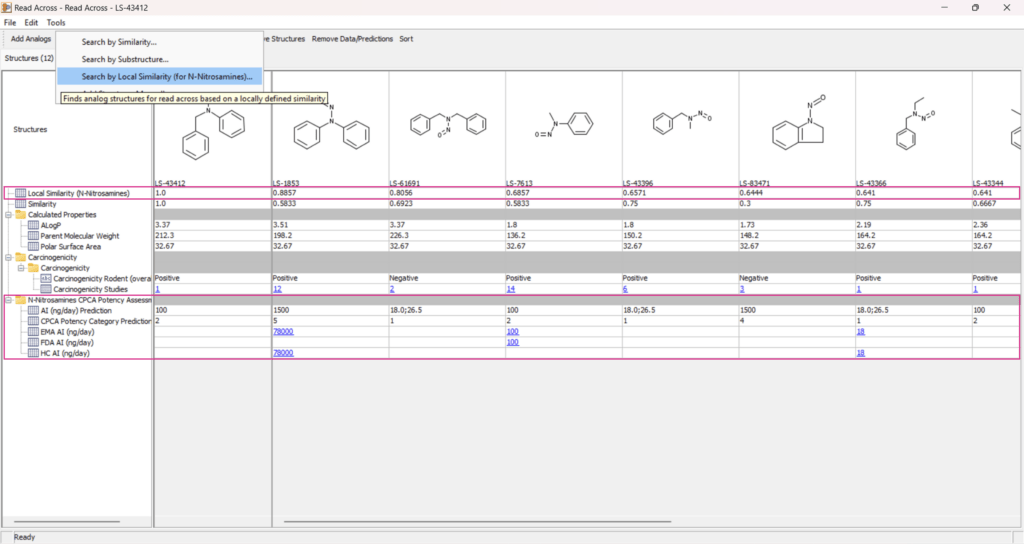
Optimizing Predictive Toxicology
The latest Leadscope Model Applier release offers more accurate predictions, improved transparency, and expanded databases to support regulatory risk assessment and computational toxicology.
Schedule a demo with us to find out more about the new features.

