KnowledgeScan™
KnowledgeScan provides robust Target Safety Assessments (TSA) to streamline biological target profiling. This service combines a translational informatics platform, automated processing, and human expertise to drive accurate and rapid TSAs for better decision-making and faster development.
Ready to make smarter, faster decisions? Request a demo today.
What is KnowledgeScan?
KnowledgeScan is a specialized TSA service designed to accelerate and improve decision-making in drug development. Instem’s expert team sources data from millions of records, using automation to reduce bias and incorporating expert human insights to ensure reliability and accuracy. By replacing time-consuming and often incomplete manual literature reviews, KnowledgeScan delivers more complete and efficient target safety assessments.


The service is highly flexible and can be applied at any stage of the drug discovery process, from evaluating potential candidates to investigating unexpected preclinical results. Services include a Full TSA and Core TSA tailored to meet your specific needs and internal resources. KnowledgeScan provides the ultimate in TSA capabilities, helping researchers tackle ambitious projects and timelines with confidence.
Key Benefits of KnowledgeScan
Saves Time
Reduced Bias
Removes Risk
Increases Quality
Expedites Development
Core Features

Powerful Visualizations

Transparent Methodology

Knowledge Gaps Identified

Pharmacology Review

Physiological Review

Toxicology & Safety Alerts

Bioinformatics Review

Systematic Anatomical Review
Why Choose KnowledgeScan?
Manually reviewing literature and toxicology datasets is time-consuming and fails to provide the detail necessary for a robust TSA. This traditional approach can leave teams uncertain about their decisions at a time when they should be moving forward confidently with a well-reviewed and strategically selected target.
KnowledgeScan transforms this process by using computer-aided data acquisition to ensure all relevant information is considered from the start, immediately providing an edge over conventional methods. Furthermore, data is reviewed by Instem’s expert team to verify accuracy, giving you greater confidence in your choices.
KnowledgeScan supports your entire drug development journey, enabling you to refine experiments and optimize processes as new internal and external data emerges. Whether you’re focusing on a single target or seeking an impartial understanding of complex signaling networks, KnowledgeScan is your trusted partner in driving smarter, faster decisions.
Customer Success Stories
The Challenge:
Haisco Pharmaceutical Group, focused on innovative drug discovery, needed to streamline its TSA process to align with its growth strategy and goals. However, limited published data on novel targets made safety analysis time-consuming and resource-intensive. An efficient, high-quality solution was essential to support rapid R&D decisions.
The Solution:
Haisco partnered with Instem to implement KnowledgeScan. By combining automated data mining, expert review, and standardized reporting, KnowledgeScan was the perfect complement to Haisco’s in-house capabilities. This approach enhanced insight into target-related risks and boosted Haisco’s TSA output.
The Results:
- Increased Efficiency – TSA production accelerated significantly and freed scientists from manual data searches.
- Improved Decision-Making – Comprehensive, curated reports enabled faster, evidence-based target evaluations.
- Resource Optimization – Internal teams focused more on analysis and less on data gathering.
By leveraging KnowledgeScan, Haisco increased TSA capacity, boosted productivity, and built a stronger foundation for confident, well-informed drug discovery decisions.

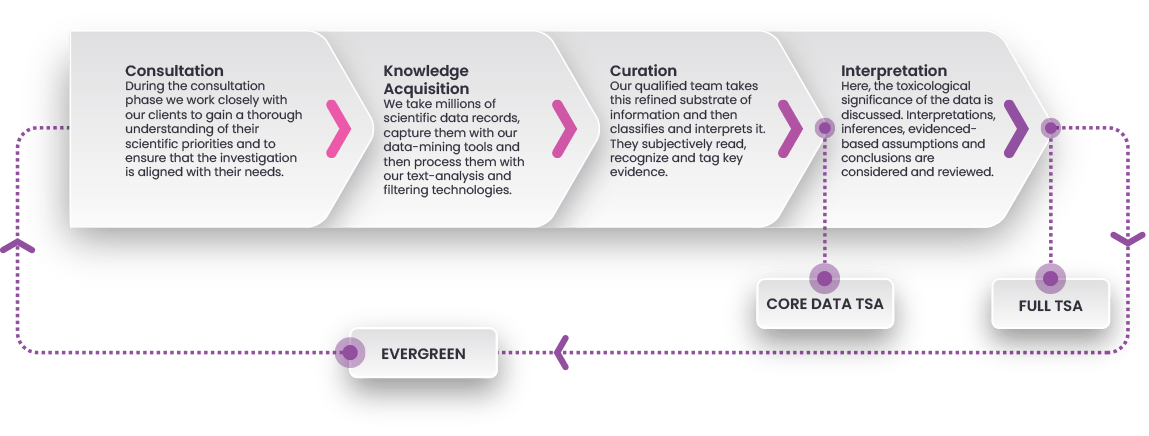
How It Works
By combining advanced data-mining technology with expert scientific insights, KnowledgeScan provides researchers with reliable, up-to-date toxicological assessments. Here’s how it works to deliver tailored and efficient target safety assessments at every stage of your drug discovery journey.
1. Consultation
2. Knowledge Acquisition
3. Curation
Our expert scientists classify, tag, and further refine the data to ensure accuracy, relevance, and scientific integrity.
4. Interpretation (Full TSA)
5. Continuous Updates (Full TSA)
Featured Resources
Our featured blog articles, white papers, and additional materials provide valuable insights, industry trends, and best practices, all aimed at helping you navigate complex safety assessment challenges and make informed decisions throughout the drug discovery process.
Frequently Asked Questions
Find answers to the most commonly asked questions about this service’s features, capabilities, and how it can support your research. Whether you’re getting started with KnowledgeScan for the first time, or looking for specific details, you’ll find the information you need right here.

