On Wednesday, October 29, 2025, a recent live webinar was led by study management experts at Instem to discuss the many challenges faced in managing preclinical animal studies and how ClimbTM is uniquely suited to address these challenges. They explored how development and updates to Climb continue to enable faster, more efficient, and more accurate animal studies. Below is an overview of that presentation.
Challenges in Preclinical Animal Studies
Preclinical study management today is increasingly complex, with many organizations operating across multiple facilities, time zones, and teams. Study directors, vivarium managers, project managers, veterinary staff, and technicians all need fast access to accurate information. Non-GLP work often involves multiple time points, interrelated procedures, and evolving protocol needs that require dynamic adjustments.
Manual scheduling and siloed tools, like paper notes and spreadsheets, are increasingly outdated. When data is scattered across notebooks, shared drives, or local spreadsheets, it becomes hard to validate whether it is accurate and up to date. In non-GLP, preclinical environments, traceability remains essential. Whether for internal governance, welfare documentation, or external review, labs must maintain clear, auditable study histories.
What is Climb?
Climb is Instem’s cloud-native software platform, purpose-built for preclinical in vivo research. It brings study management, animal management, colony management, study design, task scheduling, sample tracking, and data capture into a single, unified system.
Climb is designed with flexibility at its core. Non-GLP workflows frequently require rapid adjustments. Mid-study changes, such as adding new animals, shifting time points, and altering sampling schedules, are common. Climb can handle these changes seamlessly without compromising data integrity.
Climb sits within the preclinical stage of the drug development pipeline, supporting early discovery where rapid iteration is critical. Climb was developed by scientists, for scientists. It’s built for the full spectrum of roles involved in in vivo studies: study directors, vivarium managers, veterinary staff, technicians, data analysts, project managers, and IT teams. Through comprehensive animal tracking and information, Climb directly supports high standards of animal welfare.
“Whether you’re looking for a flexible tool for non-GLP studies, software to support vivarium management, or animal healthcare tracking and breeding, Climb is now your go-to solution.” – Chris Nichols, Senior Director at Instem
A Look Inside Climb: Demo Highlights
During the webinar, we took a look at the Climb interface to see how practical workflows drive improved study efficiency, accuracy, and coordination. If you want to see Climb in action, be sure to check out the webinar for a comprehensive demo.
When users log into Climb, they are greeted by their personalized workspace. An example from the demo is shown below, where you can see the comprehensive workspaces alongside the Insights tab, which displays animals assigned to the user along with a broad range of tasks. The Climb team showcase the breadth and scope of tasks available. Climb is therapy-agnostic, meaning a wide range of tasks and protocol types are available. Every activity and task is linked to both the responsible user and the relevant animal or study.
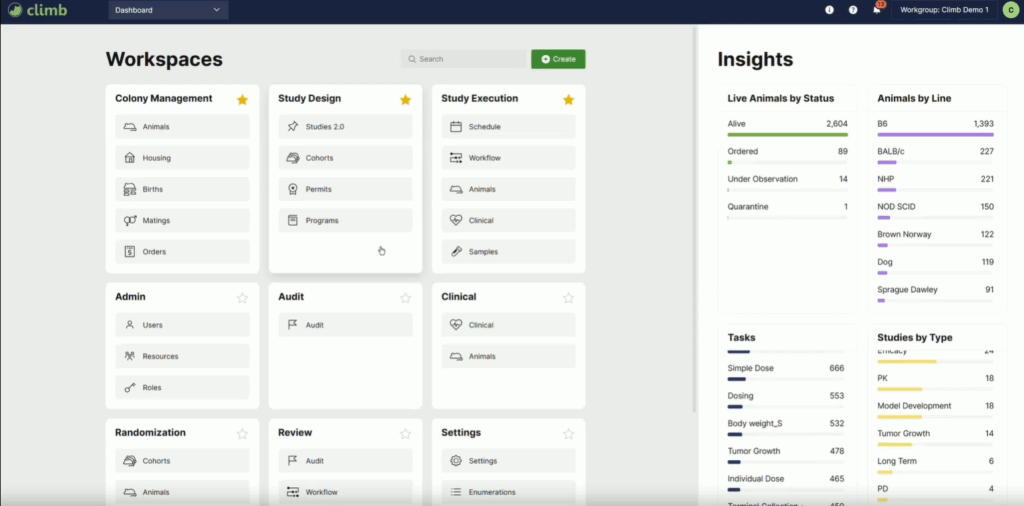
Because Climb is built cloud-first, it supports in-app messaging and delivers updates regularly. The announcement panel keeps users informed of new features, enhancements, and fixes. This rapid improvement cycle is one of Climb’s greatest strengths, ensuring the platform continually evolves with user needs.
In the demo, we see how the Study Design tab and the Study Execution tab can power in vivo studies.
Study Design
The study design workspace is highly customizable. Users can apply filters to quickly find the studies and tasks most relevant to their role, whether by permit holder, study lead, date range, or other criteria.
We use a “Lego block” analogy to illustrate how study design works in Climb. Tasks such as dosing or blood collection function as building blocks. Protocols assemble these blocks into workflows. Finally, a comprehensive study is constructed by combining multiple protocols into a unified framework.
This design supports everything from straightforward dosing studies to highly complex protocols with interdependent sampling events. Once the study is set up, Climb automatically creates placeholders for animals and generates all associated tasks, dramatically reducing setup time and minimizing the risk of configuration errors.
Users can also randomize cohorts based on defined criteria, such as body weight or tumor volume, and manage animals from birth through enrollment.
Study Execution
During study execution, Climb provides a comprehensive schedule view showing all tasks across users, teams, and time points. This ensures clarity around responsibilities and timelines. One of Climb’s standout features is dynamic rescheduling. If a procedure is delayed or a new time point is added, all dependent tasks automatically adjust. For studies involving highly sensitive timing, this automation eliminates manual recalculation and reduces the risk of downstream errors.
Data entry occurs directly within each task, and the system automatically flags non-valid or abnormal values. Climb records who performed each action, when it was completed, and any associated comments, providing full transparency and auditability.
Recent Updates and Advancements
During the webinar, Beac shared several significant enhancements released recently:
Severity scoring – Climb now supports EU and global animal welfare scoring requirements. Users can define expected severity scores per study, record actual scores with detailed metadata, and track welfare trends across an animal’s lifespan.
Study reporting improvements – A new Word-based Study Plan Report supports study design review. Meanwhile, the Excel-based Study End Result Report compiles study data, notes, animal records, clinical observations, and task information for easy analysis and review. Upcoming updates will introduce birth dates, comments, and additional metadata to enhance reporting further.
Mid-study alterations – Users can add, remove, or replace animals during an active study, for example, if they have been removed for health concerns. Climb automatically updates all related tasks, making adjustments painless.
Animal nicknames – Animals used across multiple studies can now have study-specific nicknames to support clarity and prevent confusion in analysis, especially useful for large-animal models.
Resource assignment enhancements – Bulk assignment tools make it easy to reassign tasks based on staff availability, day of the week, workload, or task type. For example, users can instantly route weekend work to weekend staff or shift tasks if a technician becomes unavailable.
These updates reflect our continuous, user-driven development philosophy, ensuring Climb grows alongside the needs of the scientific community.
Conclusion
Climb unifies data, automates schedules, and provides the flexibility researchers need in dynamic environments. Through an integrated, cloud native interface, it enables teams to work faster, more accurately, and with greater confidence. Every enhancement, release, and feature is shaped by real-world feedback from researchers and animal care teams.To see these capabilities in action, you can watch the full webinar or book a free demo to explore how Climb can support your team firsthand.


