The recent Leadscope Consultants User Group Meeting brought together Leadscope’s scientists and industry consultants to explore how Leadscope’s in silico tools are supporting drug discovery and development.
The session, which was hosted by Candice Johnson (Product Manager) and Kevin Cross (Director of In Silico Science and Business Development), showcased Leadscope’s capabilities beyond ICH M7 application.
Keynote: Read-Across Applications and Best Practices
Arianna Bassan, Co-founder of Innovatune, opened the meeting with an insightful presentation on read-across methodologies. In this keynote address, Arianna emphasized key learnings and best practices from an Instem-initiated ‘Read-across Collaborative Working Group’ that explored the application of read-across in various contexts. These insights set the stage for deeper discussions on how computational tools can support read-across assessments.
Feature Highlights Supporting Drug Development Workflows
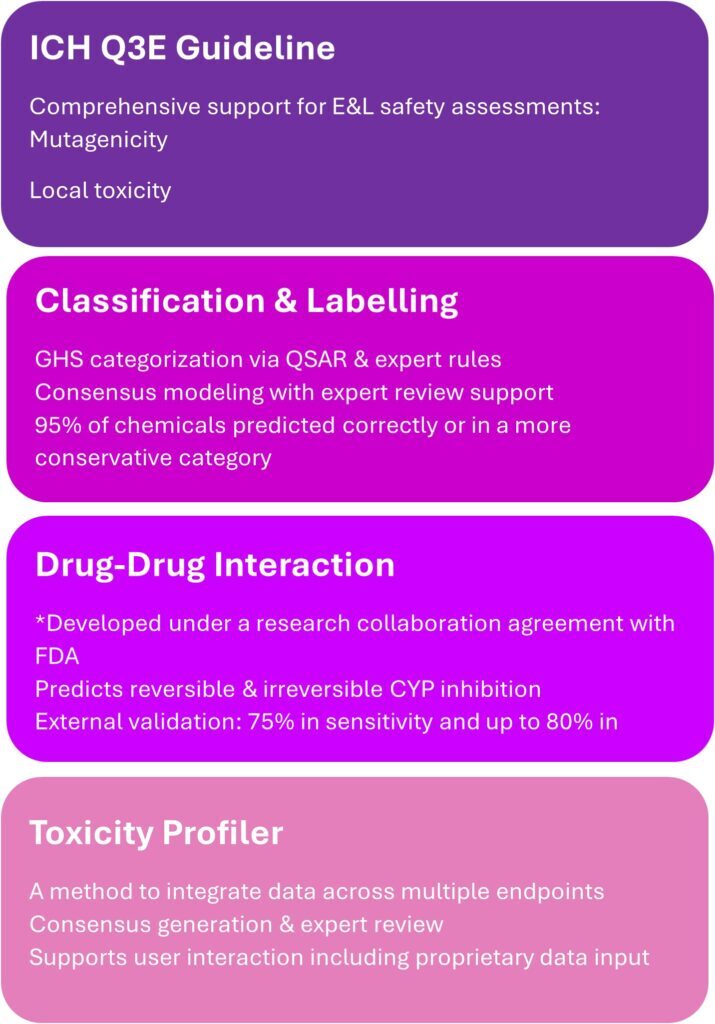
The Leadscope team presented several use cases, which can be supported by in silico assessments. Figure 1.
🧬 Drug-Drug Interaction Modeling
- QSAR models predict reversible and irreversible CYP enzyme inhibition, aligned with FDA guidance.
- Developed under a Research Collaboration Agreement with the FDA, these models offer 75% sensitivity and up to 80% negative predictivity to help assess metabolite risks.
🧪 Classification and Labelling
- Leadscope supports GHS categorization using both expert rule-based and statistical QSAR models.
- A consensus approach ensures conservative predictions, with 95% accuracy in assigning chemicals to correct or more conservative categories.
🧠 Toxicity Profiler
- A unified tool for integrating experimental data and predictions across multiple endpoints.
- Enables automatic consensus generation, manual interaction, and expert review.
Regulatory Alignment: ICH Q3E and E&L Assessments
Instem’s proactive engagement with the ICH Q3E guideline was a focal point. The guideline introduces a holistic framework for assessing extractables and leachables (E&L), and Leadscope’s tools are already supporting:
- Mutagenicity assessments via ICH M7-compliant models
- Skin sensitization predictions with high sensitivity and coverage
- Systemic toxicity evaluations using read-across and QSAR approaches to evaluate similarity
*Does not imply endorsement by the FDA
Figure 1: Summary of areas discussed during the Leadscope Consultants’ User Group meeting.
Leadscope’s transparent, expert-reviewable models and features continue to support consultant users in delivering confidence and reliable predictions for their clients.
Want to learn more or get involved?
Please contact us to speak to an expert or learn more about Instem’s In Silico solutions here.


