Accelerate Your Formulation and Dosing Workflow
Accurate and traceable information for reagents, test items, and formulations is crucial for meeting regulatory standards and maintaining research integrity in preclinical toxicology studies. As these studies grow in scale and complexity, traditional paper-based documentation systems fall short, introducing errors, inefficiencies, and compliance risks. Pharmacy Formulation, part of the Provantis suite from Instem, offers a centralized, digital solution that streamlines information management, supports multi-site collaboration, and delivers the precision and traceability required for successful regulatory submissions. This article covers everything you need to know about this powerful module, including how it can safeguard study data and reduce risk.
Reliable Formulation Data Is Key to Regulatory Success
Maintaining an accurate and detailed inventory of clinical and preclinical test items, formulations, and reagents is essential for ensuring regulatory compliance and supporting accurate submissions. This inventory must document the origin, preparation, usage, and disposal of all materials used throughout the study. Managing this data can be difficult, especially when relying on manual entry and paper-based systems, which are prone to errors and document misplacement. The challenge is further compounded when studies involve multiple teams, leading to fragmented and inconsistent datasets.
Pharmacy Formulation is a dedicated module that sits within the Provantis suite of solutions for toxicology studies, and offers a centralized platform for managing formulation data, helping researchers to overcome challenges by streamlining data entry, ensuring consistency across sites, and maintaining full traceability from receipt to disposal.
What is Pharmacy Formulation?
Pharmacy Formulation is a web-based module designed to maintain a robust inventory of test items, reagents, and formulations in toxicology studies. It is a centralized electronic information repository that tracks materials throughout their entire lifecycle, from receipt to disposal. Pharmacy Formulation helps scientists and researchers in regulated environments overcome the challenges of traditional methods, allowing them to conduct complex studies with confidence and efficiency.
Why Traditional Methods Fall Short
Traditional record-keeping methods for tracking reagents in pharmacology studies, primarily paper-based systems, are no longer adequate for the complexities of modern toxicology research. These manual processes are inefficient, prone to human error, and lack the traceability needed for GLP-compliant work.
Inaccuracies in recording can lead to formulation inconsistencies, mislabeling, and dosing errors, all of which introduce variability and undermine result reproducibility. Such issues not only risk regulatory non-compliance but may also distort a potential therapeutic’s perceived efficacy or safety. This can result in significant investment of time and resources based on flawed data. In contrast, electronic systems provide a reliable, efficient, and traceable alternative, improving data integrity, supporting compliance, and enhancing confidence in study outcomes.
Core Features and Capabilities of the Pharmacy Formulation Module
Precision and Repeatability
Pharmacy Formulation ensures precision and consistency by automating formulation calculations. It utilizes real-time data, such as body weights from the Instem InLife™ module, to optimize dosing accuracy. The system includes built-in compound confirmation before administration, reducing the risk of errors. This improves the reliability of experimental results, minimizes unnecessary costs, and helps uphold ethical standards in the treatment of animal subjects.
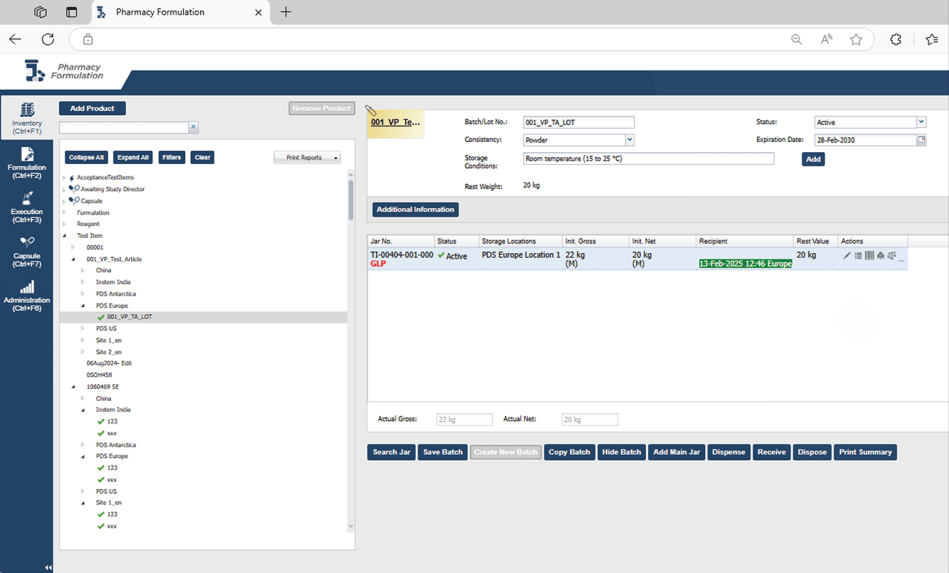
Complete Visibility and Inventory Control
The platform assigns unique barcodes to each reagent and test item, enabling easy tracking and quick searches through a dedicated database. This robust inventory system captures comprehensive details about each sample, including its geographical and on-site location, storage conditions, current status, and accountability records. Real-time inventory updates ensure authorized users have complete visibility into available reagents, simplifying management and improving decision-making around ordering and batch control. This level of oversight supports efficient and accurate inventory handling.
Seamless Integration with Provantis
Pharmacy Formulation integrates directly with the Provantis Study Protocol module, allowing automatic dosage updates for formulation preparation. This ensures experiments always follow the most current protocol, reducing the risk of discrepancies between updated instructions and ongoing work. As a result, valuable time and resources are safeguarded, and studies remain accurate and aligned with regulatory expectations and internal planning.
Secure and Paperless Workflow
Pharmacy Formulation provides research teams with a secure, GLP-compliant electronic workflow, where records are protected through strict role-based access controls. This ensures full compliance with 21 CFR Part 11 and maintains comprehensive traceability with detailed audit trails. Access is managed through the Provantis Security Hub, ensuring that sensitive information is only available to authorized on-site personnel.
User-Friendly Interface and Workflow Guidance
The module offers step-by-step guidance to researchers during formulation preparation, ensuring adherence to established protocols and reducing the risk of dosing errors caused by human mistakes or outdated instructions. It also supports localization in multiple languages and can be configured for use across various sites, enhancing collaboration and improving operational efficiency for teams working in global environments.
Conclusion
In an environment where compliance and efficiency are paramount, inventory accuracy and traceability are vital. Pharmacy Formulation provides research teams with a modern inventory management solution for toxicology studies, centralizing formulation management and integrating seamlessly with existing study protocols. It enhances data integrity, reduces errors, and ensures studies align with regulatory requirements. Pharmacy Formulation can adapt to growing teams and study complexity, making it a wise choice for future-proofing toxicology workflows.
Contact one of our experts today to request a demo and learn firsthand how Pharmacy Formulation can remove the risk from your toxicology studies.


