Safety pharmacology is a demanding yet critical phase of early drug development. It requires accuracy to ensure actionable insights and translatability, as well as efficiency to meet competitive timelines. ICH guidelines require a comprehensive battery of tests across key areas, including cardiac, respiratory, and central nervous system (CNS) safety. Achieving speed and accuracy across these domains is challenging and necessitates the complex integration of software and instrumentation. Fragmented systems and manual processing introduce errors and inefficiencies, which in turn increase risk and prolong development timelines. NOTOCORD-hem™ is a dedicated software suite from Instem that provides comprehensive coverage across experimental setups with over 160 modules and expert support. This article will cover the challenges researchers face in data acquisition for pharmacological safety studies and highlight the power of NOTOCORD-hem™ in overcoming them.
Challenges in Physiology Data Acquisition
The ICH S7A and S7B guidelines outline the recommended tests for preclinical safety of pharmaceuticals and are endorsed by major regulatory bodies, including the US FDA and EU EMA1. Addressing these recommendations requires capturing, acquiring, analyzing, and reporting large volumes of diverse data types across complex experimental setups. This often involves capturing multiple data streams from several subjects within a single experiment. For instance, cardiovascular studies can require the capture of hemodynamics, electrocardiogram (ECG) fiducial points, and arrhythmia data over extended periods. Synchronizing this data poses another challenge, as aligning key events across diverse data types is essential for drawing accurate conclusions.
Accurate and reliable data require well-planned experimental setups and a robust, reliable platform for recording, integrating, and analyzing the results. Manual data acquisition and analysis introduce errors, while limited recording capabilities can miss crucial information, impacting reproducibility. Furthermore, using software from multiple third-party providers contributes to the already high costs of drug development and introduces challenges with data integration2.
What Is NOTOCORD-hem™?
NOTOCORD-hem is a dedicated one-stop solution for preclinical safety pharmacology studies. It provides over 160 modules and applications for coverage of various experimental setups across key test areas, including cardiovascular, respiratory, and CNS assessments. NOTOCORD-hem provides real-time acquisition and analysis of crucial endpoints, as well as continuous video capture, and accommodates the most commonly used data collection methodologies, including telemetry. Large pharmaceutical companies such as Janssen and Merck all rely on NOTOCORD-hem for non-GLP and GLP-ready safety pharmacology studies. This trust highlights the critical role this solution plays in global preclinical research and its contribution to driving reliable, translatable results.
Core Capabilities of NOTOCORD-hem™
NOTOCORD-hem possesses unique features that establish it as the ultimate solution for data acquisition in preclinical safety pharmacology studies. Collectively, these features make this platform a standalone solution that covers vast experimental domains within safety pharmacology.
Comprehensive Compatibility
NOTOCORD-hem offers robust hardware compatibility, allowing it to integrate seamlessly with existing laboratory infrastructure and experimental designs. Commonly used hardware supported by NOTOCORD-hem includes:
- DSI PhysioTel telemetry
- Stellar telemetry
- Data Translation A/D card
- Primetech iPrecio Dual infusion pumps
- EMMS plethysmography
- HD digital video cameras
A straightforward user interface makes it simple for researchers to establish complex acquisition and analysis workflows (Fig. 1). This helps to minimize setup and downtime, and once a suitable workflow has been established, automated capabilities ensure it is performed consistently across different cohorts and studies, improving accuracy and reproducibility.
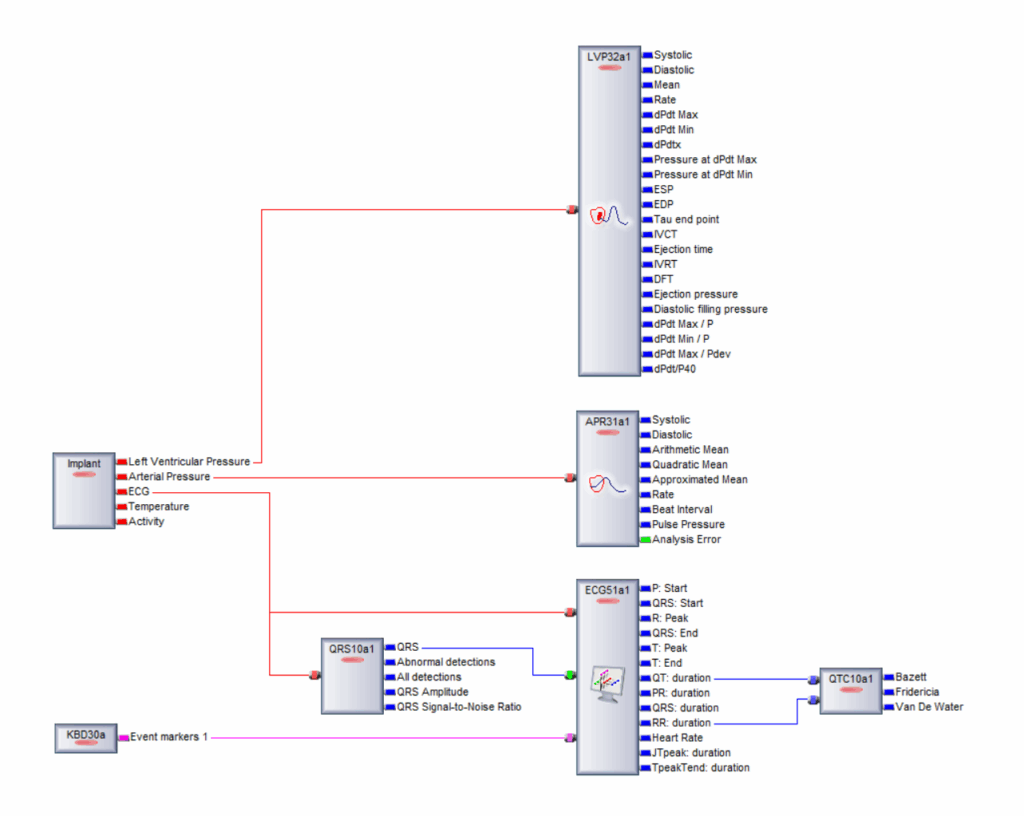
Figure 1. NOTOCORD-hem’s user-friendly interface makes it easy for researchers to configure complex experimental workflows.
Real-Time Analysis and Visualization
The analyzer modules in NOTOCORD-hem provide researchers with real-time insights into their data acquisition, which they can visualize through the straightforward interface. When coupled with synchronization across multiple output channels, this helps provide real-time understanding of experimental outcomes, a crucial ability when establishing new protocols and deciding if experiments should be ended early.
High Reliability and Data Integrity
We designed NOTOCORD-hem to support early-stage research and GLP applications, ensuring compliance with data acquisition and privacy requirements. NOTOCORD-hem is fully compliant with 21 CFR Part 11 requirements. User identification is managed by Windows Group Policy and Active Directory. Traceability is ensured through an electronic audit trail, and each action is automatically recorded and cannot be modified. All administrative actions are recorded, and modifications are logged into the Windows Event Log.
Flexible and Scalable
NOTOCORD-hem is a comprehensive solution that eliminates the cost of sourcing from multiple third parties. It is a suitable option for both small laboratories and large pharmaceutical companies conducting studies across various sites. The solution easily adapts to the pace of early research as protocols rapidly evolve while providing a robust platform for performing established protocols with no variation.
How NOTOCORD-hem™ Empowers Researchers
NOTOCORD-hem empowers researchers and organizations to achieve time- and cost-effectiveness for small and large-scale safety pharmacology studies.
Timesaving
NOTOCORD-hem allows researchers to collect and process vast amounts of data, with Microsoft Excel macros for automated export and reporting. Its intuitive interface minimizes the need for extensive staff training and allows for faster implementation.
Cost-saving
The platform offers over 160 modules and applications to ensure comprehensive coverage of experimental setups, thereby reducing the need to source solutions from multiple third-party vendors. Its hardware compatibilities remove the need for expensive purchases, allowing researchers to work with what they have.
Accuracy
Automated workflows ensure reproducibility and accuracy, giving researchers more confidence in their decisions and ultimately enhancing the translatability of clinical trials.
Trusted
The world’s largest pharmaceutical companies trust NOTOCORD-hem for their GLP data acquisition workflows.
Conclusion
NOTOCORD-hem™ removes complexity from preclinical safety pharmacology by unifying data acquisition, analysis, and reporting in one platform. It empowers researchers to deliver faster, more accurate results through seamless data acquisition and analysis, which is trusted by the most prominent players in the pharmaceutical industry. NOTOCORD-hem™ is the ultimate standalone solution for today’s competitive drug discovery environment, allowing researchers and organizations to achieve cost and time efficiencies with zero compromise.
Contact one of our experts today to learn how NOTOCORD-hem™ can support your preclinical safety pharmacology studies, no matter the scale or complexity.
References
1. ICH Official web site : ICH. Accessed March 14, 2025. https://www.ich.org/page/quality-guidelines
2. Sertkaya A, Beleche T, Jessup A, Sommers BD. Costs of Drug Development and Research and Development Intensity in the US, 2000-2018. JAMA Netw Open. 2024;7(6):e2415445. doi:10.1001/jamanetworkopen.2024.15445


